XJTU research team makes progress in aqueous supercapacitors
The research uses betaine to regulate electrode/electrolyte interphase property.
Aqueous supercapacitors have attracted attention due to their excellent capacitive characteristics, high safety, and environmental friendliness. However, the narrow electrochemical stability window of water (1.23V) reduces the energy storage density of the devices, limiting their practical applications.
To address this issue, Professor Li Lei's team from the School of Materials Science and Engineering at Xi'an Jiaotong University (XJTU) has developed a new strategy. They use zwitterionic betaine to regulate the solid-liquid interface properties between activated carbon and aqueous electrolytes, thereby improving the working voltage and electrode charge storage capacity of the devices, leading to a significant increase in energy density.
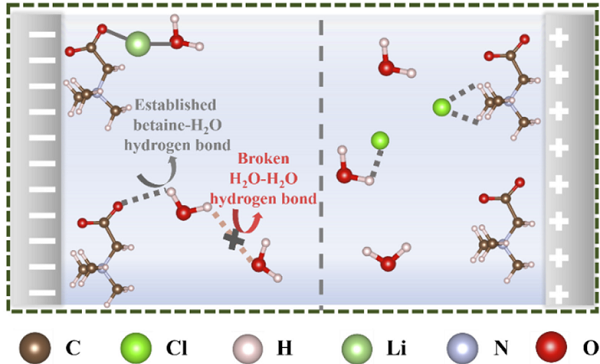
Betaine evenly coats the surface of activated carbon, preventing direct contact between the aqueous electrolyte and activated carbon. On one hand, betaine adsorbs water from the electrolyte, forms new hydrogen bonds, and disrupts the original hydrogen bonds of water, thereby reducing the activity of water near the activated carbon. This results in a significant increase in the working voltage of the device from 1.0V to 1.4V.
On the other hand, betaine has a stronger adsorption capacity for electrolyte ions than activated carbon, leading to a substantial increase in device capacitance from 21.35Fg-1 to 27.73Fg-1 at 1Ag-1. In addition, this strategy significantly reduces the leakage current and voltage drop of the device.
The above results were published in Energy Storage Materials under the title Regulating electrode/electrolyte interphase property via betaine to turbo supercapacitor energy storage.

