XJTU team proposes AORFBs with high stability and low cost
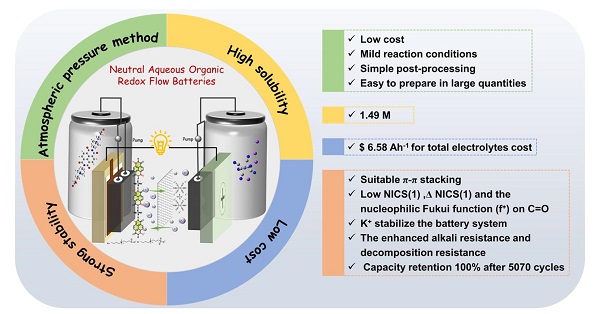
The atmospheric pressure method for synthesizing AORFBs is proposed by Professor He's team.
Professor He Gang's team from the Frontier Institute of Science and Technology at Xi'an Jiaotong University (XJTU) has proposed an innovative ionic synergistic strategy to synthesize highly stable naphthalene diimide zwitterion derivatives [(CBu)₂NDI, (SPr)₂NDI, (SPrOH)₂NDI] using an atmospheric pressure method.
Single-crystal structural analysis revealed that electrostatic interactions between intermolecular side chains in (CBu)₂NDI induce a parallel-staggered π-π stacking configuration (spacing: 3.45 Å, displacement angle: 42.8°), effectively suppressing molecular aggregation in highly reactive radical states while maintaining superior aqueous solubility under radical conditions.
Computational studies involving Nucleus-Independent Chemical Shift [NICS(1)] and Fukui function analyses demonstrated that anion incorporation significantly inhibits OH⁻-induced nucleophilic degradation of electrolyte materials. Single-point energy calculations further elucidated the stabilizing role of K⁺ ions in supporting electrolytes during battery cycling.
This strategy achieved a remarkable aqueous solubility of 1.49 M for (CBu)₂NDI, enabling a total electrolyte cost as low as $6.58·Ah⁻¹. The assembled battery exhibited negligible capacity decay over prolonged cycling at 2 M electron concentration, demonstrating exceptional stability. These advancements significantly propel the development of high-performance neutral aqueous organic redox flow batteries (AORFBs) and highlight their potential for large-scale commercial deployment.
The rapid growth of renewable energy has intensified demand for low-cost, long-lasting, and safe large-scale energy storage technologies to achieve carbon neutrality. While naphthalene diimide (NDI) derivatives are promising candidates owing to their extended π-π conjugation and two-electron storage capabilities, practical applications remain hindered by OH⁻-triggered decomposition of side chains/imide rings during cycling and radical-induced viscosity surges.
Existing NDI materials modified with hydrophilic groups still face critical challenges of insufficient molecular stability and limited battery cycle life. Consequently, developing NDI-based electrolytes that combine high stability with low cost constitutes the core scientific challenge for commercializing AORFBs.
This groundbreaking research, titled Synergistic ionic modification strategy enhances the stability of naphthalene diimide zwitterions for cost-effective aqueous organic redox flow batteries, was recently published in the internationally authoritative journal National Science Review.

